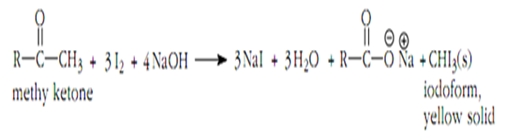iodoform test for aldehyde and ketone Iodoform mechanism
The Haloform Reaction and Iodoform Test are valuable tools in the field of chemistry. These reactions are particularly useful for identifying and characterizing methyl ketones. Methyl ketones, which have the chemical formula RCOCH3, are organic compounds that contain a carbonyl group bonded to a methyl group. By utilizing the Haloform Reaction and Iodoform Test, chemists can easily determine the presence of methyl ketones in a substance.
Haloform Reaction and Iodoform Test play a crucial role in the identification of methyl ketones. They are widely used techniques in the field of chemistry due to their effectiveness and ease of execution. These reactions rely on the characteristic reactivity of methyl ketones with halogens, such as chlorine, bromine, and iodine, to produce haloforms.
The first step of the Haloform Reaction involves the halogenation of the methyl ketone. In the presence of a halogen and a base, such as sodium hydroxide (NaOH), the methyl ketone undergoes nucleophilic substitution. The halogen replaces the carbonyl oxygen, resulting in the formation of a halogenated compound.
Next, the halogenated compound reacts with hydroxide ions to form a carboxylate ion. This carboxylate ion then undergoes further oxidation, resulting in the release of a halide ion and the formation of a haloform. The haloform can be detected by its distinct odor and yellow precipitate.
The Iodoform Test is a specific example of the Haloform Reaction that uses iodine as the halogen source. This test specifically identifies methyl ketones, as they react readily with iodine to form iodoforms. This reaction is particularly useful in distinguishing methyl ketones from other aldehydes and ketones.
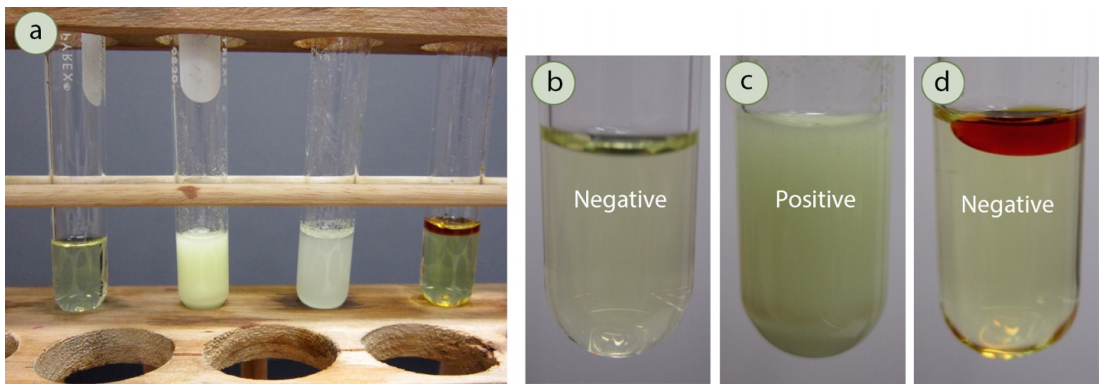
The Iodoform Test is straightforward to perform. A sample suspected of containing a methyl ketone is mixed with a solution of iodine in sodium hydroxide. If a yellow precipitate forms, it indicates the presence of a methyl ketone. The formation of iodoform is a positive test result.
In conclusion, the Haloform Reaction and Iodoform Test are essential tools for chemists in the identification and characterization of methyl ketones. These reactions allow for the easy detection of methyl ketones through the formation of haloforms, such as iodoform. By utilizing these techniques, chemists can confidently identify the presence of methyl ketones in various organic compounds.
If you are searching about Iodoform Test Description you’ve visit to the right web. We have 5 Images about Iodoform Test Description like Haloform Reaction and Iodoform Test - Chemistry Steps, How is aldehyde distinguish from ketone Give chemical equation and also Iodoform Test For Alcohols : Why does ethyl isopropyl ketone not give. Here it is:
Iodoform Test Description
 byjus.comiodoform mechanism
byjus.comiodoform mechanism
Iodoform Test For Alcohols : Why Does Ethyl Isopropyl Ketone Not Give
 kumisawixh.blogspot.comiodoform ethyl alcohols libretexts chem precipitate methyl ketone
kumisawixh.blogspot.comiodoform ethyl alcohols libretexts chem precipitate methyl ketone
How Is Aldehyde Distinguish From Ketone Give Chemical Equation
 www.meritnation.comOne Part Of Chemistry: Reactions Of Aldehydes, Ketones And Phenols
www.meritnation.comOne Part Of Chemistry: Reactions Of Aldehydes, Ketones And Phenols
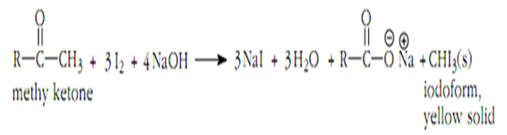 1chemistry.blogspot.dkiodoform ketone test acetaldehyde reaction chemistry part methyl
1chemistry.blogspot.dkiodoform ketone test acetaldehyde reaction chemistry part methyl
Haloform Reaction And Iodoform Test - Chemistry Steps
 www.chemistrysteps.comiodoform reaction methyl ketones chemistry chemistrysteps
www.chemistrysteps.comiodoform reaction methyl ketones chemistry chemistrysteps
How is aldehyde distinguish from ketone give chemical equation. One part of chemistry: reactions of aldehydes, ketones and phenols. Iodoform ketone test acetaldehyde reaction chemistry part methyl