tollens test for aldehyde and ketone Tollens benzaldehyde mechanism
Tollens’ Test is a commonly used chemical test in the field of chemistry to detect the presence of aldehydes. It is named after Bernhard Tollens, a German chemist who developed this test in the late 19th century. This test involves the oxidation of aldehydes using Tollens’ reagent, which is a solution of silver nitrate (AgNO3) in aqueous ammonia (NH3).
The Principle Behind Tollens’ Test
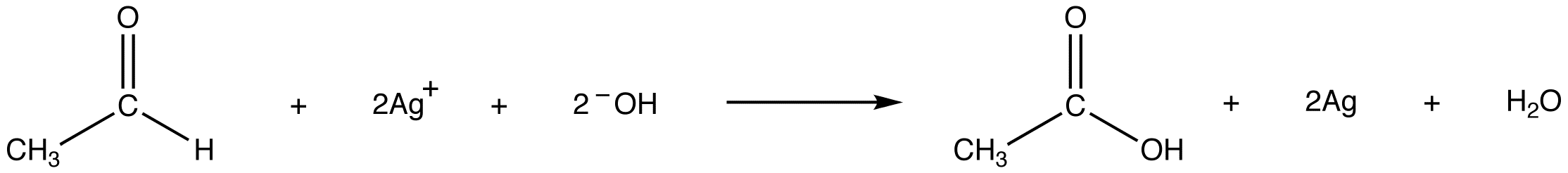 The principle behind Tollens’ Test is the ability of aldehydes to undergo oxidation to form carboxylic acids in the presence of Tollens’ reagent. The reagent acts as an oxidizing agent, converting the aldehyde into a carboxylate anion and reducing the silver ion (Ag+) in silver nitrate to metallic silver (Ag).
The principle behind Tollens’ Test is the ability of aldehydes to undergo oxidation to form carboxylic acids in the presence of Tollens’ reagent. The reagent acts as an oxidizing agent, converting the aldehyde into a carboxylate anion and reducing the silver ion (Ag+) in silver nitrate to metallic silver (Ag).
Performing Tollens’ Test
To perform Tollens’ Test, a small amount of the substance suspected to contain aldehydes is mixed with Tollens’ reagent and heated. If the substance is an aldehyde, a silver mirror is formed on the inner side of the test tube. This silver mirror is a characteristic observation of a positive Tollens’ Test.
Applications of Tollens’ Test
Tollens’ Test is particularly useful in detecting the presence of aldehydes in organic compounds. It is commonly used in the identification of various aldehydes, including formaldehyde, acetaldehyde, benzaldehyde, and others. By performing Tollens’ Test on an unknown organic compound, chemists can narrow down the possibilities and determine if the compound in question contains aldehydes.
Limitations of Tollens’ Test
Although Tollens’ Test is a simple and effective method for detecting aldehydes, it has some limitations. One major limitation is that ketones do not give a positive Tollens’ Test. Since ketones cannot be oxidized by Tollens’ reagent, no silver mirror is formed. This specificity makes it a useful test to differentiate between aldehydes and ketones.
Oxidation of Aldehydes and Ketones
Another important aspect of aldehydes and ketones is their ability to undergo oxidation reactions. Aldehydes can be oxidized to carboxylic acids, while ketones are relatively resistant to oxidation due to the absence of an active hydrogen atom. Oxidation reactions convert the carbonyl group of aldehydes into a carboxylate functional group.
The oxidation of aldehydes and ketones is a significant reaction in both organic and biological chemistry. In organic synthesis, oxidation reactions are often used to synthesize carboxylic acids from aldehydes or other suitable precursors. In biological systems, the oxidation of glucose to produce energy is an example of an oxidation reaction involving aldehydes.
Overall, Tollens’ Test and the oxidation of aldehydes and ketones play important roles in the field of chemistry. They provide valuable information about the presence of aldehydes in various compounds and the reactivity of functional groups. Understanding these concepts is essential for the identification and characterization of organic substances and their chemical properties.
If you are looking for Oxidation of Aldehydes and Ketones - Chemistry LibreTexts you’ve visit to the right place. We have 5 Pictures about Oxidation of Aldehydes and Ketones - Chemistry LibreTexts like Chem 211 - Tests for Aldehydes and Ketones, Oxidation of Aldehydes and Ketones - Chemistry LibreTexts and also Tollens’ Test: Definition, Example, and Mechanism. Read more:
Oxidation Of Aldehydes And Ketones - Chemistry LibreTexts
chem.libretexts.orgtollens test mirror silver reaction aldehydes oxidation ketones aldehyde positive chemistry negative used side wikipedia left figure right libretexts science
Tollens’ Test - Chemistry LibreTexts
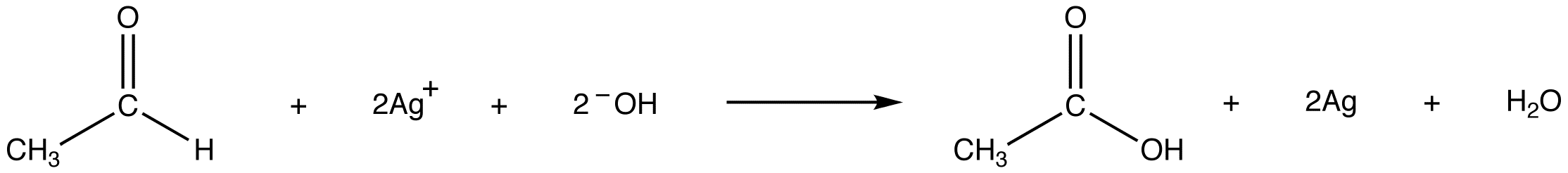 chem.libretexts.orgTollens’ Test: Definition, Example, And Mechanism
chem.libretexts.orgTollens’ Test: Definition, Example, And Mechanism
 www.chemistrylearner.comtollens mechanism
www.chemistrylearner.comtollens mechanism
Chem 211 - Tests For Aldehydes And Ketones
 academics.wellesley.edualdehyde aldehydes test tollen tollens silver ketone mirror tests ketones glucose chemistry nitrate ammoniacal chem fructose definition appendix
academics.wellesley.edualdehyde aldehydes test tollen tollens silver ketone mirror tests ketones glucose chemistry nitrate ammoniacal chem fructose definition appendix
Tollens’ Test: Definition, Example, And Mechanism
 www.chemistrylearner.comtollens benzaldehyde mechanism
www.chemistrylearner.comtollens benzaldehyde mechanism
Tollens’ test. Tollens’ test: definition, example, and mechanism. Tollens test mirror silver reaction aldehydes oxidation ketones aldehyde positive chemistry negative used side wikipedia left figure right libretexts science